Molecular Transformation Pathways from Plastic to Fuel
The conversion of plastic waste into usable fuel is a thermochemical process that fundamentally alters polymeric structures. Unlike mechanical recycling, which reshapes plastic without changing its chemical composition, thermal degradation breaks down long-chain hydrocarbon molecules into smaller, energy-rich compounds. A pyrolysis plant serves as the reactor environment for this controlled molecular transformation, turning heterogeneous plastic inputs into valuable liquid and gaseous fuels through depolymerization, cracking, and condensation mechanisms.
Depolymerization of Synthetic Polymers
The primary chemical transformation begins with the depolymerization of plastics. Most plastic waste streams—polyethylene (PE), polypropylene (PP), and polystyrene (PS)—consist of long hydrocarbon chains formed through addition polymerization. Under elevated temperatures (typically 350°C–500°C) in an oxygen-deprived plastic pyrolysis reactor, the polymer chains absorb thermal energy and reach their activation energy threshold.
As the temperature rises, covalent bonds between monomer units weaken and begin to break. This process, known as random scission, produces a complex mixture of radicals, oligomers, and low-molecular-weight hydrocarbons. The specific bond dissociation pathways are influenced by the polymer type and the residence time in the reactor.
Thermal Cracking and Molecular Fragmentation
Once the polymeric backbone is destabilized, thermal cracking intensifies the degradation process. Cracking reactions cleave C–C and C–H bonds, producing alkanes, alkenes, and aromatic compounds. These reactions are highly endothermic and can occur via free-radical mechanisms, especially in the absence of catalysts.
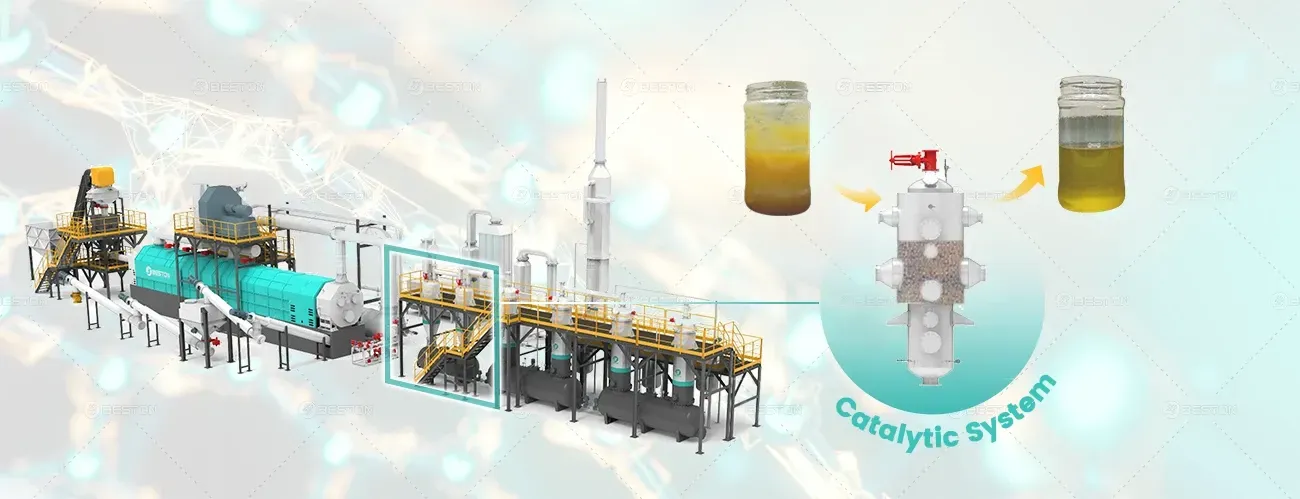
In a plastic into fuel machine, the reactor design (e.g., rotary kiln, fluidized bed, or auger type) determines heat distribution and molecular residence time, directly impacting the product composition. Shorter residence times favor lighter hydrocarbons and gas formation, while longer durations can promote secondary cracking of intermediates into lower-boiling-range compounds.
The gaseous phase contains hydrogen, methane, ethylene, and butane, while the condensable vapor stream yields pyrolysis oil—comprising paraffins, olefins, naphthenes, and aromatics in varying proportions.
Condensation and Phase Separation
As the vapor exits the high-temperature zone, it passes through a series of condensers in a controlled cooling process. This step selectively condenses heavier hydrocarbons into liquid fuel fractions while allowing non-condensable gases to be recovered for combustion or storage.
The resulting pyrolysis oil exhibits a variable composition, typically containing C5–C20 hydrocarbons with heating values ranging from 38–42 MJ/kg. The oil may contain traces of oxygenates, sulfur compounds, and halogenated derivatives depending on the feedstock purity. Further refining may involve distillation, hydroprocessing, or catalytic upgrading to produce transportation-grade fuels.
Non-condensable gases such as hydrogen, carbon monoxide, and light hydrocarbons are often recirculated into the pyrolysis plant to fuel the reactor, enhancing energy efficiency and lowering operational costs.
Solid Residue and Char Formation
In addition to fuel products, the process generates solid carbonaceous residue—commonly referred to as char. This by-product consists of unconverted carbon, inorganic fillers, and additives present in the original plastic. While its calorific value is lower than that of pyrolysis oil, char can be used in industrial heating or further processed into activated carbon, depending on composition.
Certain reactor configurations may also be equipped with dechlorination or filtration systems to capture problematic elements like PVC-derived hydrogen chloride, thus protecting the integrity of downstream systems and meeting emission standards.
Influence of Polymer Type on Conversion Outcomes
The molecular transformation pathway varies by plastic type. Polyethylene and polypropylene yield high liquid fuel ratios due to their saturated hydrocarbon structure. Polystyrene predominantly forms aromatic hydrocarbons, particularly styrene monomer. Polyvinyl chloride (PVC) and polyethylene terephthalate (PET), if present, introduce complications such as corrosive by-products and require pretreatment or segregation to prevent process instability.
Optimizing feedstock composition in the pyrolysis plant is essential for maximizing fuel yield and minimizing secondary contamination. Sorting technologies and chemical compatibility analysis play a critical role in ensuring product uniformity and economic viability.
In thermochemical terms, the transformation from plastic to fuel is a decomposition–recombination sequence governed by temperature, pressure, and feedstock chemistry. The pyrolysis plant acts as the controlled environment in which synthetic polymers are dismantled at the molecular level and reassembled into usable energy carriers. Through precise process control, waste plastic becomes a source of liquid hydrocarbons, supporting both waste reduction and alternative fuel production in a carbon-constrained economy.



