Enhancing Thermal Efficiency in Plastic Pyrolysis Reactor
In pyrolysis processes, thermal efficiency plays a vital role in determining the overall effectiveness of the reactor and the quality of the products generated. Plastic pyrolysis is no exception. A high level of thermal efficiency can significantly reduce operational costs, improve energy utilization, and increase product yield, making the pyrolysis process more economically viable. Optimizing the thermal efficiency of a plastic pyrolysis reactor involves various strategies, from improving heat transfer mechanisms to utilizing waste heat. This article explores effective ways to achieve higher thermal efficiency, thereby maximizing the performance and profitability of the pyrolysis operation.
Optimizing Reactor Design
The design of the plastic pyrolysis reactor is one of the most critical factors influencing its thermal efficiency. Enhancing the design for better heat retention and transfer can have a direct impact on the overall energy consumption of the process.
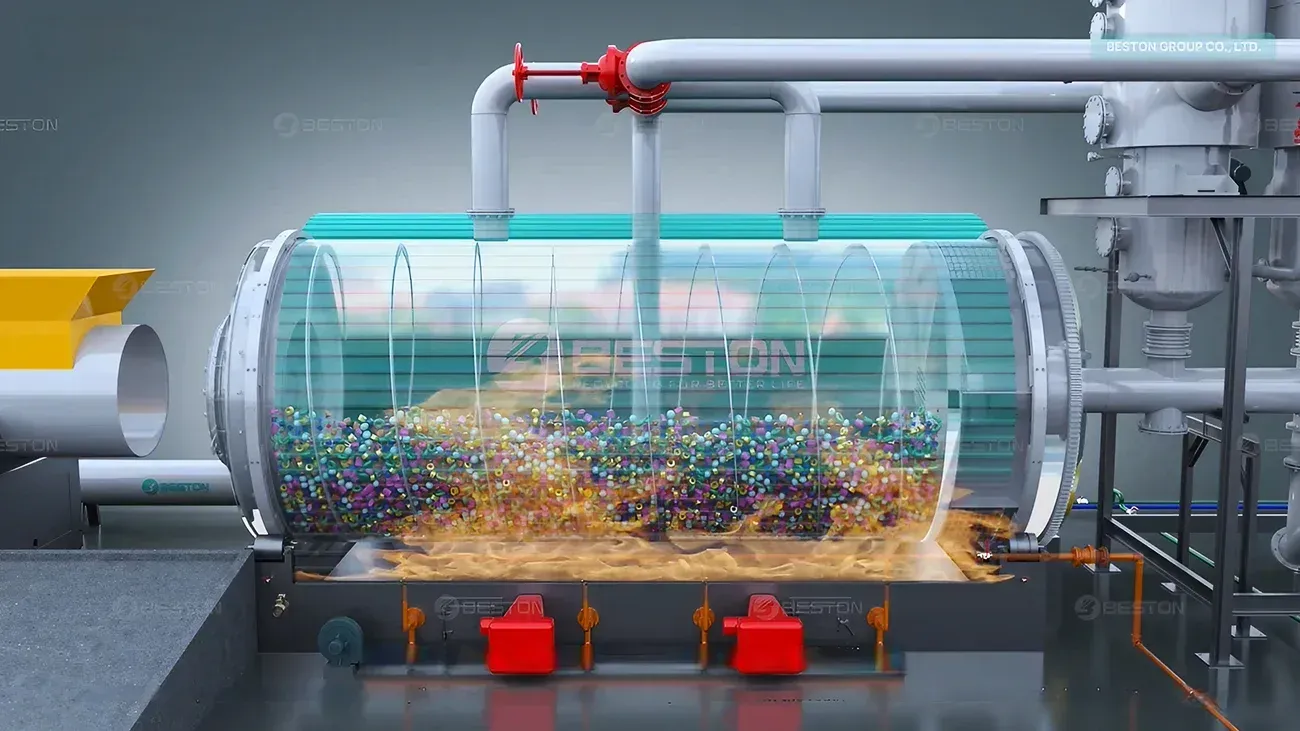
- Double or Multi-Chamber Reactors: Many modern pyrolysis reactors incorporate double or multi-chamber designs. These systems allow for more effective heat circulation and provide more space for the heat to penetrate the feedstock. As the waste plastic enters the reactor, the multiple chambers enable better heat distribution, ensuring more uniform temperatures throughout the material. This reduces energy losses and ensures that the feedstock is exposed to optimal temperatures for the thermal decomposition process.
- Insulation: Proper insulation is crucial for maintaining high thermal efficiency. By ensuring that the reactor is well-insulated, heat losses to the environment are minimized. This not only saves energy but also stabilizes the temperature inside the reactor, improving the consistency of the pyrolysis process. High-quality insulation materials, such as ceramic fiber or mineral wool, can provide effective thermal barriers, keeping the heat within the reactor and ensuring optimal operating conditions.
- Heat Recovery Systems: Heat recovery is a crucial element in improving thermal efficiency. Incorporating a heat recovery system, such as a heat exchanger, can significantly reduce energy consumption. In a typical plastic pyrolysis reactor, much of the heat is produced during the pyrolysis process and can be captured and reused to pre-heat the incoming feedstock. This reduces the reliance on external energy sources and increases the overall thermal efficiency of the reactor.
Managing Temperature Control
Precise temperature control is essential to the pyrolysis process, as the breakdown of plastics into useful products depends on maintaining the right thermal conditions.
- Automatic Temperature Regulation: Pyrolysis reactors can benefit from automated temperature control systems, which allow for precise regulation of internal temperatures. By using sensors and advanced control systems, the reactor can maintain a stable temperature, minimizing fluctuations that can cause inefficiencies. Maintaining optimal temperatures ensures that the plastic feedstock undergoes thermal decomposition at the most efficient rate, without the waste of energy due to overheating or underheating.
- Temperature Profiling: Dividing the pyrolysis reactor into multiple temperature zones can also help to optimize the thermal efficiency. By implementing different temperature settings for each zone, the reactor can ensure that the feedstock receives the appropriate level of heat at different stages of the pyrolysis process. This enables a more controlled breakdown of plastics, improving the overall yield of bio-oil, gas, and carbon black, and reducing energy waste.
- Advanced Heating Systems: Using advanced heating methods, such as indirect heating or electromagnetic heating, can also improve thermal efficiency. Indirect heating systems reduce the loss of energy to the environment and provide a more uniform heat distribution within the reactor. Electromagnetic heating, on the other hand, allows for more direct and efficient energy transfer to the feedstock, making the pyrolysis process faster and more energy-efficient.
Utilizing Waste Heat
Pyrolysis reactors generate significant amounts of waste heat during the process, and this heat, if not utilized efficiently, represents a lost opportunity for increasing thermal efficiency. Using waste heat to supplement energy needs within the reactor can drastically improve overall performance.
- Pre-heating Feedstock: Waste heat can be used to pre-heat incoming plastic feedstock before it enters the pyrolysis reactor. This step reduces the energy required to reach the desired reaction temperature, saving fuel or electricity. In this way, the reactor can operate with lower energy inputs while achieving the same or better output.
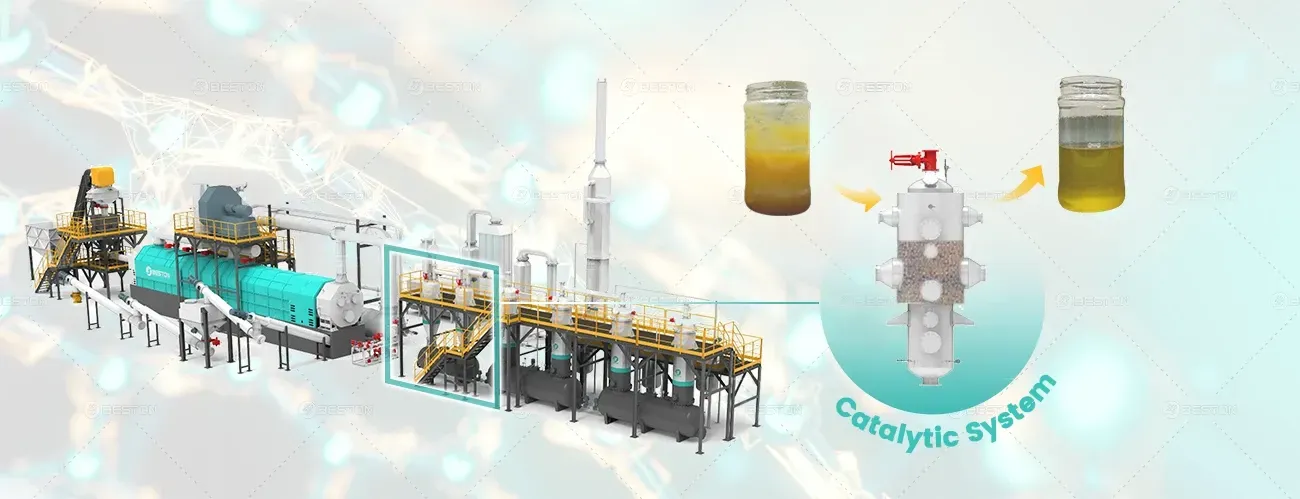
- Syngas Utilization: The syngas produced during the pyrolysis process can be used as a secondary energy source to power the reactor. By utilizing syngas in the reactor’s burner, operators can reduce dependency on external energy supplies. This process of self-sustaining heat generation through the use of syngas significantly increases the thermal efficiency of the reactor and reduces operational costs.
- Co-generation of Power: Another method of utilizing waste heat is through co-generation, where waste heat is captured and converted into electrical power. In this setup, the pyrolysis plant can generate its own electricity, reducing the need for grid power. This not only improves thermal efficiency but also enhances the sustainability of the pyrolysis operation by lowering its carbon footprint.
Improving Heat Transfer
Efficient heat transfer within the reactor is essential for maintaining thermal efficiency. The quality of the heat exchange between the heating elements and the feedstock directly impacts how well the reactor operates.
- Optimized Reactor Geometry: Adjusting the geometry of the reactor can enhance heat transfer efficiency. For example, incorporating spiral or helical designs in the reactor walls increases surface area contact, thereby improving the transfer of heat from the walls to the feedstock. This design ensures that more of the heat produced is effectively used for the pyrolysis process, reducing energy waste.
- Enhanced Stirring Mechanisms: In some reactors, the feedstock is stirred during the pyrolysis process to ensure better heat distribution. Using advanced stirring mechanisms that create uniform mixing of the plastic materials can significantly improve the overall heat distribution within the reactor, ensuring that all materials are uniformly exposed to the required temperature.
Advanced Materials and Coatings
The material composition of the reactor plays a pivotal role in its thermal efficiency. Special coatings and materials that enhance heat retention or improve heat conductivity can make a considerable difference.
- Reactor Lining: Lining the interior of the reactor with materials that are highly resistant to heat can improve both durability and thermal efficiency. High-performance refractory materials or heat-resistant ceramics are often used in the construction of reactors to ensure long-term stability and minimal energy loss.
- Thermal Coatings: Applying advanced thermal coatings to the reactor surfaces can further reduce heat loss. These coatings reflect heat back into the reaction chamber, ensuring that more energy is retained within the system and less is lost to the surrounding environment.